School season has started
If you have any homework that you need help with, you can share it here
The only rule is the same rule when it comes to helping in coding: we dont give out direct answers
Good luck to everyone
School season has started
If you have any homework that you need help with, you can share it here
The only rule is the same rule when it comes to helping in coding: we dont give out direct answers
Good luck to everyone
I need help with chemistry
like everything with chemistry
Oooh i love chemistry
What topic are you learning now?
period table stuff ![]()
periodic trends, covalent and ionic bonds, anions, cations…
I can help it’s rly easy. Go onto to Google and press the lil search button and put how to [insert what u need to do]
Every element after 83 (technically 82[1]) radioactive.
because element 83, bismuth, has a very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very very long half-life of 19.2 quintillion years ↩︎
The majority of chemical elements with an atomic number above 82 have no stable isotopes (they’re typically radioactive)
See the image below, the numbers on each element are the number of stable isotopes (variations with a different number of neutrons, but the same number of protons and electrons) of that element.
…marmite I thought you were taking a break from the discourse
no just from the PM
how could my monkey brain allow the “days visited” number for the past year to go below 365?
when does your break end we miss you :(((
2 days if my memory serves me correctly
two days meaning today and tomorrow or tomorrow and Monday?
Eh I left at 7pm UTC on the 23rd
So I guess 7 days from then would be 2pm CDT on the 30th
come back to ussssssss
soon
fun on-topic fact: the word “homework” starts with the letter h
Assuming you are using learning Bohr s atomic model:
Bohr claimed that an atom is made of a nucleus (protons and neutrons) and that electrons revolve around the nucleus in circular orbits (called shells)
He claimed that electrons fill a shell before moving onto another shell
In the first circular orbit, we can only fit 2 electrons. From the second shell and on, we can fill each of them with up to 8 electrons
this is an example of the nitrogen atom. it has 7 electrons. it filled the first shell with 2 electrons, leaving only 5 electrons for the next shell.
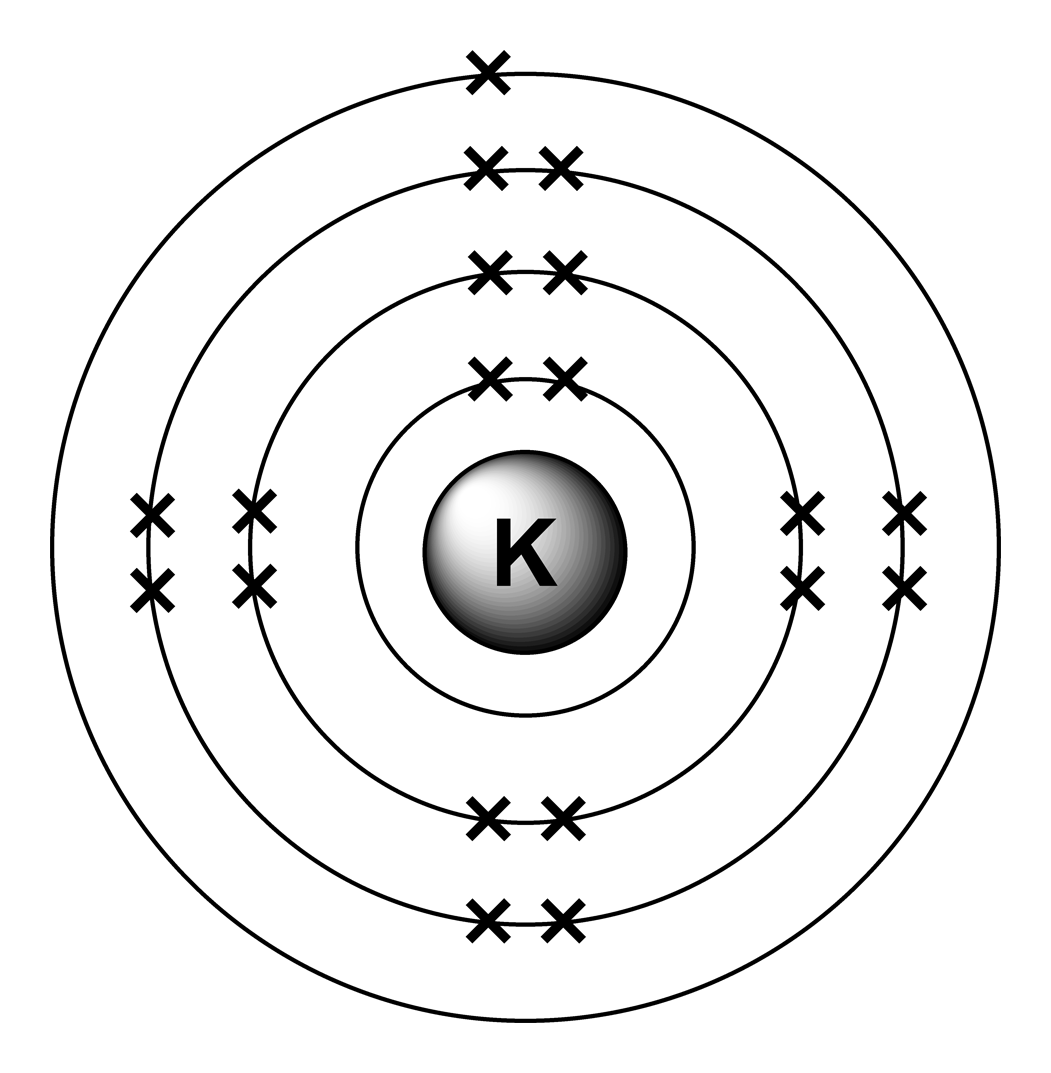
this is another example: the atom of potassium
as you can notice, the electrons filled the first shell with 2 electrons, the second and third shell with 8 electrons each, and the 19th electron was left for a fourth shell.
Important
In an atom, if we look at the outer most shell (aka the last shell), we can know if the atom can make a reaction with another atom.
How do we know? We check if the shell is full to capacity or not.
for example, I shared a picture of the nitrogen atom above. in the last shell, there are only 5 electrons. meaning we have vacancy (emptiness) for 3 more electrons to fill the shell.
this tells us that this atom is prepared to take 3 electrons in a reaction.
if you look at the last group of the periodic table, you will realize that the elements’ last shell is full, meaning they will not get into a reaction
this is an example of argon, a noble gas (an element in the last group of the periodic table)
the x marks are the electrons
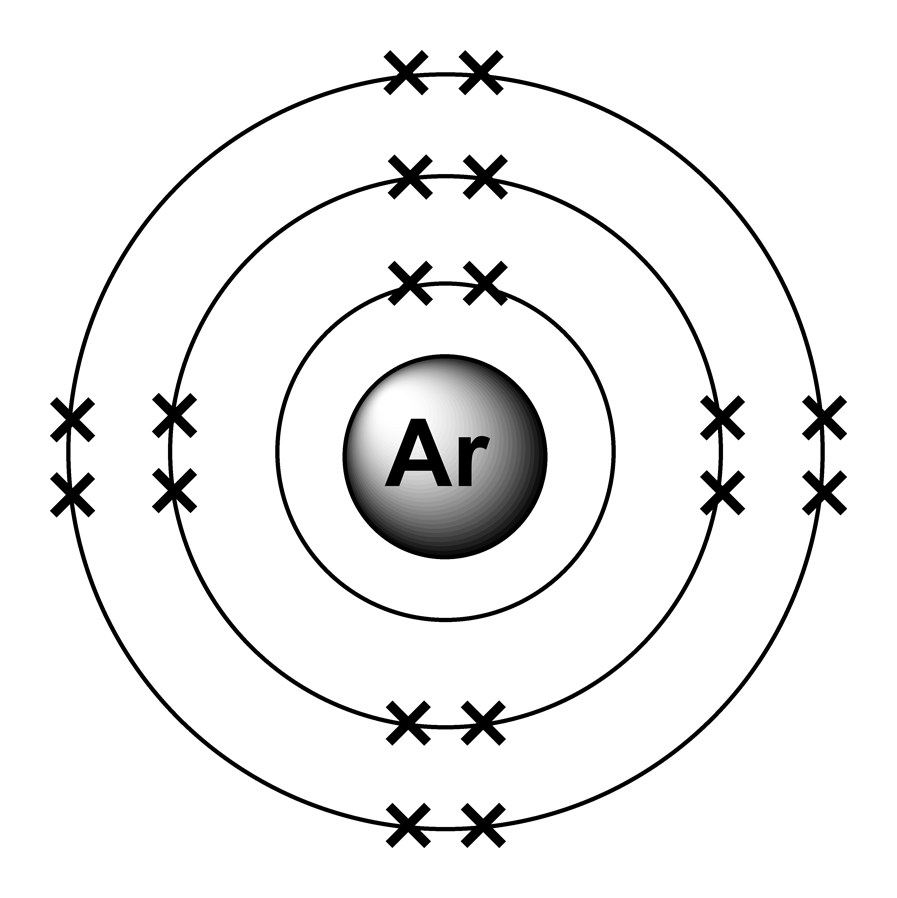
as you can see, there are 8 electrons in the outer most shell. this means that argon cannot have a reaction with other elements. it has filled all the shells it needs and decided to become an introvert.
lets go back to the examples of nitrogen and potassium
as we have said, nitrogen has 5 electrons in its second shell. it can go into a reaction because it is not completely filled. the easiest way to make the outer most shell complete is for nitrogen to take 3 more electrons from another element to make the outer most shell complete (it was 5/8 electrons, we added 3 electrons, it became 8/8 → complete)
how about potassium?
potassium’s outer most shell is filled with only one electron. is it easier for potassium to find 7 more electrons to become more stable/complete? or is it easier to just give away its last electron?
of course the last electron’s removal is much easier. so when potassium wants to go intro a reaction with another element, it gives away its not so precious electron to gain more stability/complete its outermost shell.
to sum up, if an atom’s outermost shell has less than 4 electrons, it tends to give away the electrons in the reaction. if it has more than 4 electrons, it tends to take electrons from the other element
before you continue, if you have any question ask. it is important to understand this part before moving onto the upcoming one
now that we understand how the atoms do business with electrons, lets see what the different namings of these businesses are.
lets talk about salt. salt’s chemical formula is NaCl → Sodium Chloride
this means that sodium and chlorine underwent a reaction together.
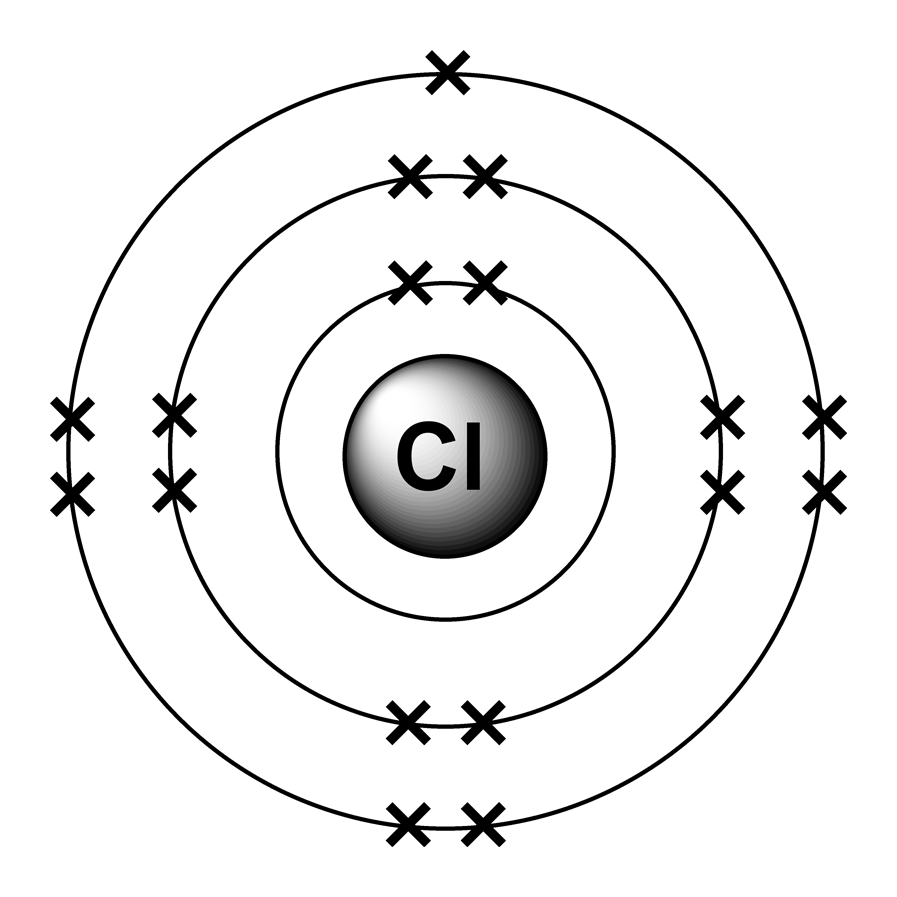
this is chlorine’s atom. as you can see, it has 7 electrons in the outermost shell. it needs 1 more electron
this is sodium’s atom. as you can see, it has 1 electron in the outermost shell. it wants to leave/give away this electron to become more stable
hold on, sodium can give this electron to chlorine and they will both be complete! and they will boil happily ever after in our cooking.
so this is exactly what happens. sodium gives its electron to chlorine; completing chlorine’s outermost shell and discarding its own last shell leaving it with 2 complete shells (the green circle disappears and sodium only has the red and blue circles
and we get salt
this is the formation of an ionic bond
sometimes, in nature, a sodium atom gets hit by radiation. this radiation might hit its only electron in the outermost shell, so it loses it. when the atom’s positive and negative charges are not equal, the atom’s name becomes an ion.
once sodium loses an electron, it would have 11 protons but only 10 electrons. this shows us that now the atom has more positives than negatives, making it positively charged. because the difference is only one charge, and when we want to represent this atom, we write a tiny + on the top right of Na. Na+, but the plus is smaller and a little bit more up.
this indicated that it has lost one electron, or that the protons are more than the electron by one.
positively charged ions are called cations. this means that Na+ is a cation.
lets talk about another example, chlorine. if chlorine was just existing, and a lost electron found its way to it, it would complete its outermost shell. as you remember, chlorine tends to take an extra electron to complete the shell.
if we compare the proton number with the electron number of the chlorine ion, we would find that the electrons are more than the protons, making it negatively charged.
this means that this ion is negatively charged. in this case, we write it Cl-, and of course the minus is closer to the top.
negatively charged ions are called anions
so these are ions, ionic bond, anions and cations
if you have any questions pleeeeaase don’t hesitate to ask. these will pop up again later on and you wont be able to understand the upcoming topics without these.
God willing, I will be explaining the covalent bond asap
![]()